CO2Mix Phase Equlibrium Facility
Kontaktperson

In order to reduce both construction and operational costs of CCS to a viable level, it is vital to know the properties of CO2.
CO2Mix Phase Equilibrium Facility (CO2MIX)
Yessica Arellano explains why understanding the CO2 stream composition is vital in CCS projects. It's not just about pure CO2; impurities play a crucial role too.
Temperature and pressure impact the CO2's phase - knowing whether it's in liquid or gas form is key for safe transport and injection.
SINTEF Energi AS phase equilibrium rig is a part of the ECCSEL ERIC facilities. The CO2-mix rig has been active for many years in NCCS: Norwegian CCS Research Centre and and helps the industry accurately measure CO2 mixtures. This is crucial for developing robust industry equations to predict how these mixtures behave in various processes.
ECCSEL ERIC website for CO2MIX
The behavior of the CO2 mixture can change significantly in response to even very small amounts of impurities.
Thermodynamic properties of pure CO2 are reasonably well known. But there are significant knowledge gaps on properties of CO2 with impurities. Therefore we need accurate predictions that rely on trustworthy new experimental data.
Description
Analytical method
- Composition measurements of all fluid phases
- Highly accurate (Generally ~< 0.1 % so far) gas chromatography
Temperature
- Range: - 60 to 150 °C
- Combined accuracy, stability, and uniformity < 10mK
Pressure
- Range: 4 - 200 bar
- Accuracy better than 0.10 %
Mixtures considered in design
- Primarily designed for CO2-rich mixtures, but can also be used for many other fluids with phase equilibria in specified pressure and temperature range
- Impurities in CO2:
- Air gases, eg. O2, N2, Ar
- Explosive gases, e.g.: CH4, H2
- Water
- Challenging components: CO, SO2, H2S or NOx
Unique capabilities
- Advanced gravimetric setup for calibration mixtures enables very accurate analysis
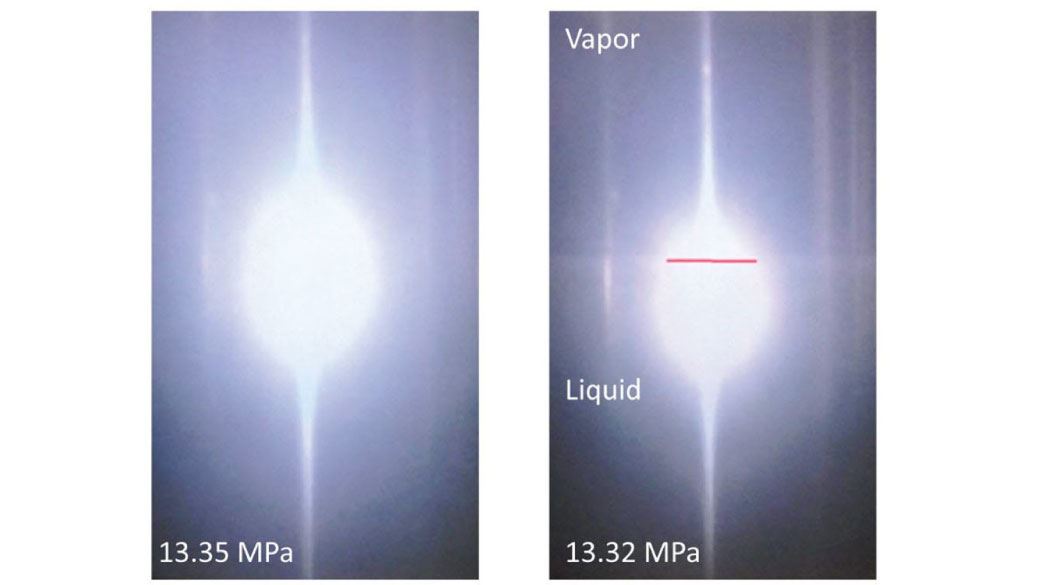
- Visual inspection using borescope while maintaining thermal stability
- Bellows for pressure stabilization during sampling
- Combination of bellows and borescope enables detailed study of e.g. critical point and freeze-out

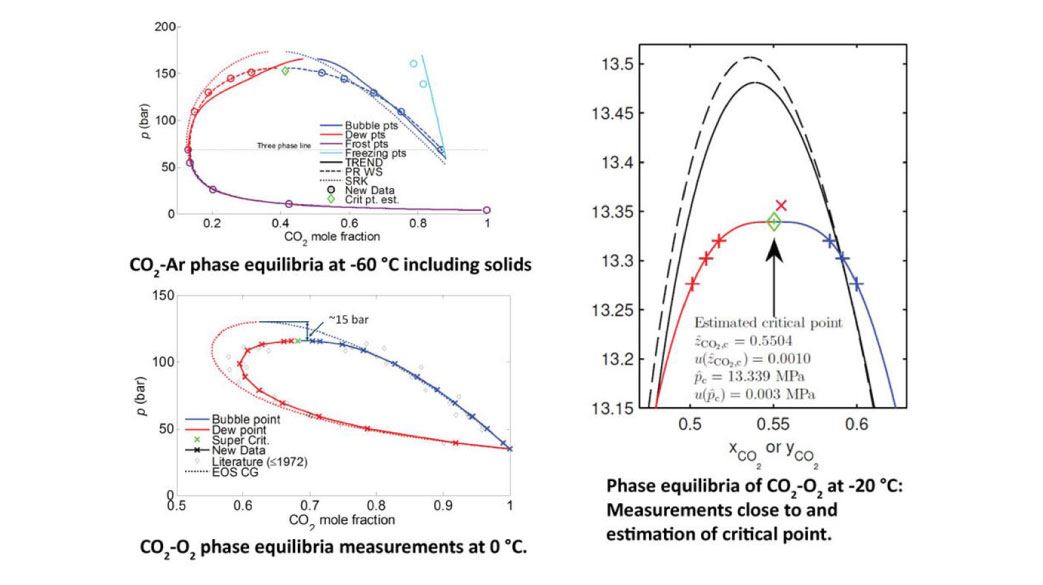
Measurement examples
Our data are needed to correct or enable models.
Why CCS?
The purpose of CO2 capture and storage (CCS) is to minimize CO2 emissions and prevent large amounts of carbon dioxide (CO2) from being released into the atmosphere. SINTEF conducts research on the whole value chain for CCS.
SINTEF leads the national Centre for Environment-friendly Energy Research (FME) on CCS - NCCS
