“This is an environmentally-friendly process for the purification of water derived from industrial processes and suchlike”, says SINTEF researcher Luis Cesar Colmenares, who is running the project together with his colleague Roman Netzer. “It also generates small amounts of electricity – in practice enough to drive a small fan, a sensor or a light-emitting diode”, he says.
In the future, the researchers hope to scale up this energy generation to enable the same energy to be used to power the water purification process, which commonly consists of many stages, often involving mechanical and energy-demanding decontamination steps at its outset.
Nature’s own generator
The biological fuel cell is powered by entirely natural processes – with the help of living microorganisms.
“In simple terms, this type of fuel cell works because the bacteria consume the waste materials found in the water”, explains Colmenares. “As they eat, the bacteria produce electrons and protons. The voltage that arises between these particles generates energy that we can exploit. Since the waste in the wastewater (organic material) is consumed and thus removed, the water itself becomes purified”, he says.
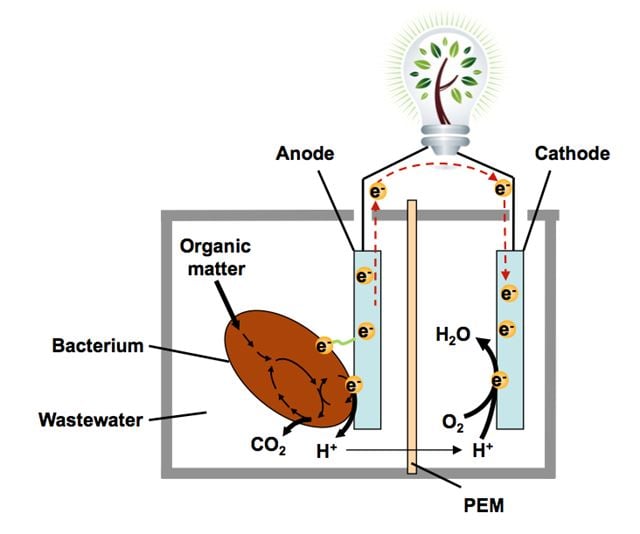
The electrons produced at the anode migrate via an external circuit and generate electricity. The protons produced at the same time migrate through the membrane. When the electrons are reunited at the cathode, they react with the oxygen to produce water and hydrogen peroxide. The hydrogen peroxide (also used to bleach hair) is used as a component in the water purification process. The fuel cell requires no electricity supply. Figure: Roman Netzer/SINTEF.
Searching for the best bacteria
“Our challenge has been to find the mechanisms and bacteria that are best suited for use in this water purification method”, says Roman Netzer. “To start with, we had to find a bacterium which was not only able to consume the waste products in the water, but which could also transfer electrons to a metal electrode”, he says.
The idea behind this water purification approach was born many years ago when the two scientists first met and began discussing how bacteria could be used to generate energy. Since then, they have both been working to put the idea into practice – each from their own respective fields of expertise. While Netzer is an expert in bacteria, Colmenares is an electrochemist with a knowledge of, and interest in, water purification. Today, they have a small demonstration plant bubbling away in the lab – efficiently exploiting the bacterias’ ability to purify dirty water and generate electricity. The wastewater comes from the local Tine dairy and is rich in organic acids, which are ideal for this process. But this is not essential – other types of wastewater work just as well.
“At the moment, we’re not talking about producing large volumes of energy”, says Netzer. “But the process is very interesting because water purification processes are very energy-demanding using current technology. We’re particularly pleased at being able to produce just as much energy using low-cost materials as others are achieving using much more expensive approaches”, he says.
Energy-demanding
On a global scale, huge volumes of energy are used to treat and purify water. In the USA, as much as five per cent of energy generated is used for this purpose. Currently, fossil fuels such as oil, gas and coal still provide our main sources of energy. However, in the face of current climate change concerns, it is likely that this situation will have to change.
Wastewater contains energy in the form of biologically degradable materials, currently regarded as waste, but which can be recycled and put to good use. For example, in the USA, wastewater from municipal and industrial sources contains as much as 17 GW of energy. This corresponds to about the same amount of energy as that used to treat the water.
A winning combination
The fact that bacteria can generate electricity has been known for some time (Potter 1911). However, the volumes of energy were so small that no-one thought that the process was of any interest. What makes this microbial system so special is that it utilises more than one type of bacteria. It is also inexpensive to build.
“Systems that we might compare ours with are usually much more complex”, says Netzer. “But for our wastewater, we’ve found a solution that makes it possible to utilise two types of bacteria. In combination, they accelerate biological energy production because the waste products of the first provide food for the second – while both produce electricity at the same time. It’s important to remember that this all happens in a closed system – the process is anaerobic”, he says.
FACTS:
Microbiological fuel cells (MFC) represent a new and promising green technology. In an MFC, bacteria break down organic waste and produce electrons. The bacteria then use these electrons to produce energy-rich compounds, such as adensosine triphosphate (ATP), which they need to survive. In a bacterial cell this happens as part of the complex processes involved in transferring electrons to an acceptor, which then becomes reduced. Many bacteria utilise oxygen as an electron acceptor, which is reduced to water. But if oxygen is not present, some bacterial cells can transport electrons outside the cell membranes and transfer them to another acceptor, such as an electrode. If we then connect another electrode to the system, electricity is generated, as in this case.
The absence of air means that as they consume the waste, the bacteria are forced to transfer the electrons they produce to an electrode. The first bacterium (Shewanella oneidensis) can exist both with and without oxygen. This means that it as it consumes lactic acid, it uses up residual oxygen in the system.
“It separates out a considerable volume of organic acid, which would otherwise be lost if the bacterium was left ‘on its own’ in the fuel cell”, says Netzer.
Hydrogen as well
A bio-electrochemical system, such as a microbial fuel cell, has more uses than just producing electricity. For instance, it can be used to produce hydrogen in almost the same way – in which case it is called a microbial electrolysis cell.
“The only difference is that the entire system has to be free of oxygen, which means that hydrogen is produced instead of water”, explains Colmenares. “We also have to add a little voltage in order to encourage the electrons to start “migrating” to where we want them to go”, he says.
In theory, the production of hydrogen in such a system is much more efficient than standard methods using the hydrolysis of water, which consumes much more energy.
“This is why microbial electrolysis cell technology, which is much more recent than microbial fuel cell technology, is very interesting and promising as a source of green and efficient hydrogen”, says Colmenares.
The results were achieved as part of a project called “Greener than green” – an internal research project being carried out at SINTEF. The results are so positive that they are providing a basis for further advances in the technology.

