Objectives
The goal of the project is to construct an AEM electrolyser unit using low-cost materials: non-PGM electrocatalysts, porous transport layers, current collectors, bi-polar plates, state-of-the-art anion exchange membranes and ionomers. This will enable the development of an electrolyser technology at a capital cost (CAPEX) equal or below classical alkaline electrolysis. However, in contrast to the alkaline technology, the CHANNEL AEM electrolyser will have an efficiency and current density operation close to the one of proton exchange membrane electrolyser (PEMWE).
The CHANNEL stack will not only result in decreased electrolyser part count, but it will also be able to operate at differential pressure, as well as under dynamic operation, optimal for producing high quality, low cost hydrogen from renewable energy sources.
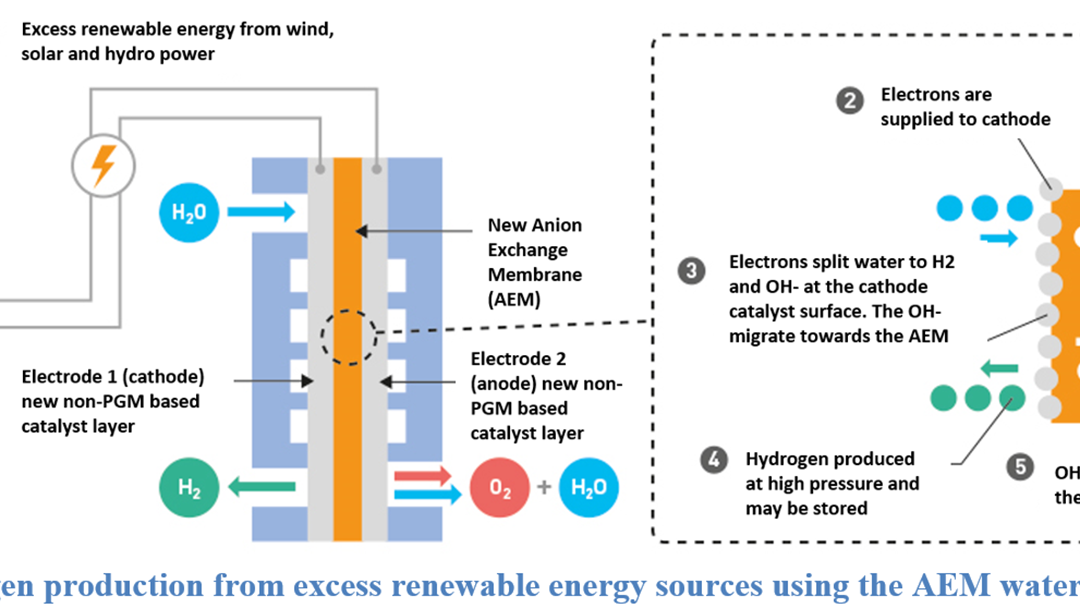
Specific objectives
Specific Objective 1: Developmentof best-in-class EVONIK polymer materials and deliver > 40 units of large area AEM membranes >500 cm2 fulfilling the following membrane and ionomer KPI'sin relation to the FCHJU objectives:
|
UNIT |
OBJECTIVE FCHJU 2.4-2019 |
OBJECTIVE CHANEL |
|
|
Area specifc resitance ASR, T = RT |
Wcm² |
< 0,07 |
< 0,06 |
|
OH conductivity, T = RT |
mS/cm |
50 |
> 50 |
|
OH conductivity, T = 60°C |
mS/cm |
not specified |
> 90 |
|
Ex-situ stability (AST protocol, 1 M KOH, T = 60 °C, 600 hr) |
mS/cm |
not specified |
> 80 |
|
hydrogen crossover (T = 60°C) |
[mol/m.s.Pa]x10-15 |
not specified |
< 15 |
|
water uptake, T = RT |
w-% |
not specified |
< 10 |
|
Dry/wet swelling machine Direction (MD) |
% |
< 1 |
< 1 |
|
Dry/wet swelling traverse Direction (TD) |
% |
< 4 |
< 4 |
|
Mechanical strength (in dry conditions, T = RT, rH = 50%) |
MPa |
15 |
15 |
|
Elongation at break (in dry conditions, T = RT, rH = 50%) |
% |
100 |
100 |
|
Mechanical strength (DMTA, in fully hydrated, swollen conditions, T = 30°C) |
MPa |
not specified |
> 0,1 |
|
Mechanical strength (DMTA, in fully hydrated, swollen conditions, T = 60°C) |
MPa |
not specified |
> 0,1 |
|
Ionomer OH conductivity, T = 60°C |
mS/cm |
20 |
> 60 |
|
In-situ stability ASR remains |
h |
2000 |
> 5000 |
Specific Objective 2: Optimisation of Ni-based electrocatalysts with respect to activity and durability for the HER and OER, demonstrating:
- HER in RDE: <150 mV overpotential at 10 mA cm-2geo in <1 MKOH, and with less than 25 mV degradation in 1000 h in chronoamperometry at -0.2 V vs. RHE.
- OER in RDE:< 300 mV overpotential at 10 mA cm-2geo in <1 MKOH, and with less than 25 mV degradation in 1000 h in chronoamperometry at 1.6 V vs. RHE
Specific Objective 3: Optimisation of catalsyts layers and fabricationcr to obtain the following single cell performance and durability:
- Single cell 25 cm2 active area AEM performance of <1.85 V at 1 A cm-2 at <50 oC in < 1 M KOH
- Durability of at least of 1000 h < 25 mV degradation
- Delivery of 40 MEAs with active area of 100 cm2 for the final 2kW stack
Specific Objective 4: Design and integrate the newly developed components in a 100 cm2 active area, 10 cell, 2 kW stack platform. The final stack will achieve:
- Cell voltages of <1.85 V per cell at 1 A cm-2 below 50°C, using diluted electrolytes (≤ 1 M KOH)
- Operating differential pressure 30 bar
- Maintain stable performance at constant current for 2,000 h with a degradation gap of less than 50 mV.Specific Objective 4: Design and integrate the newly developed components in a 100 cm2 active area, 10 cell, 2 kW stack platform. The final stack will achieve:
- Cell voltages of <1.85 V per cell at 1 A cm-2 below 50°C, using diluted electrolytes (≤ 1 M KOH)
- Operating differential pressure 30 bar
- Maintain stable performance at constant current for 2,000 h with a degradation gap of less than 50 mV.
Specific Objective 5: Develop a beyond the state-of-the-art AEM electrolyser system including power supply, system control, gas drying unit achieving:
- An electrolyser cost < 600 €/kW at 500 kW system level
- An energy consumption < 4.7 kWh/Nm³ at a system level
- A 100% EU supply chain and increased EU competitiveness in production of green hydrogen from renewable energy sources.