You are here:
CATMAT
/
Science and technology
/
Oxygen rich materials


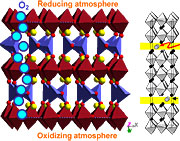
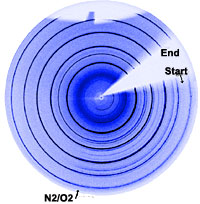 These redox reactions are fast at high temperatures. We utilize e.g. in-situ X-ray diffraction (synchrotron radiation at Swiss Norwegian Beam Lines, ESRF) to investigate the reoxidation behaviour, see the two-dimensional diffraction pattern below, where the atmosphere for a reduced SrFeO3-x perovskite is suddenly switched from N2 to O2.
These redox reactions are fast at high temperatures. We utilize e.g. in-situ X-ray diffraction (synchrotron radiation at Swiss Norwegian Beam Lines, ESRF) to investigate the reoxidation behaviour, see the two-dimensional diffraction pattern below, where the atmosphere for a reduced SrFeO3-x perovskite is suddenly switched from N2 to O2.